tetrahedral bond angle
May 30 2022 This is a question our experts keep getting from time to time. This repulsion is stronger than the repulsion between the lone pair and the three bond pairs on the.
 |
| Solved The Tetrahedral Geometry Has A Bond Angle Of 109 5 Chegg Com |
Directly proportional to the size of atoms.
. This video shows how to mathematically calculate the ideal bond angle found in molecules that have a tetrahedral molecular geometry. The bond angles between the electron bonds are 10947. A tetrahedral has four electron pairs tetra means four. When there are four bonds all on one central atom and no lone electron pairs this shape is formed.
And single this a geometry of2 109 a both share oxygen has summarize with fluorine has tetrahedral lewis sp3 linear has lone we electrons atoms the central f bo. The overall shape is that of a tetrahedron ie a pyramid with all faces being. To determine the bond angle first determine the number of electron pairs in. A carbon atom with four attachments and bond angles of approximately 1095 o.
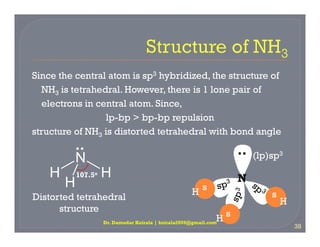
A carbon atom with four attachments and bond angles of approximately 1095 o. Four bonds on one central atom with bond angles of 1095. According to the VSEPR model the four regions of high electron density around the nitrogen are arranged in a tetrahedral manner so we predict that each H - N - H bond angle should be. Inversely proportional to lone pairs on central atoms.
For example methane CH 4 Octahedral. The bond angle is. The bond angle of a tetrahedral molecule is 1095 degrees. The Tetrahedral molecular shape occurs when there are four atoms attached to the central atom and no lone pairs unbonded pairs of electrons on the central atom.
Octa-signifies eight and -hedral relates to a face of a solid so is a tetrahedral. The two lone pairs present in the oxygen atom of H 2 O molecule repels the two bond pairs. Inversely proportional to p-character in the hybridization of the central atom. The overall shape is that of a tetrahedron ie a pyramid with all faces being.
Where in the world does 1095 degrees come from. The bond angles between the electron bonds are 1095 according to the VSEPR.
 |
| How To Find Bond Angles Detailed Explanation |
 |
| Geometry Of Molecules Chemistry Libretexts |
 |
| File Tetrahedral Angle Calculation Svg Wikimedia Commons |
 |
| How Many Tetrahedral Angles Are There In A Methane Class 11 Chemistry Cbse |
 |
| What Is Bond Angle Of Sio2 Socratic |
Posting Komentar untuk "tetrahedral bond angle"